Description
Diclazepam
Product information
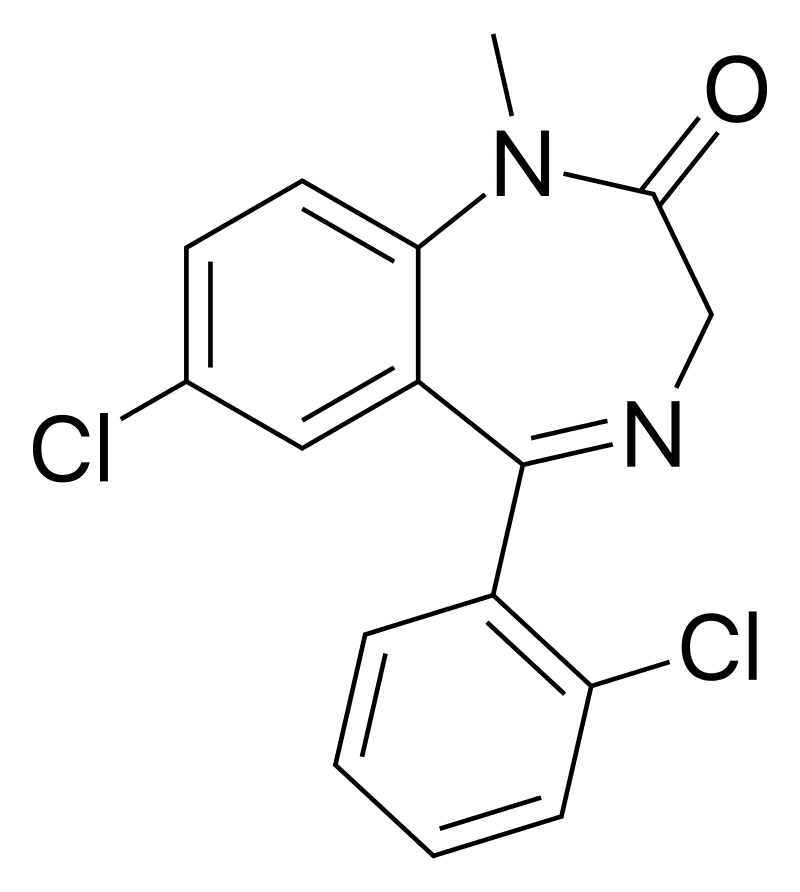
IUPAC-name 7-Chloro-5-(2-chlorophenyl)-1-methyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one
Synonyms 2′-Chlorodiazepam, Ro 5-3448, 2′-chloro-diazepam
Formal name 7-
Cas number 2894-68-0
Formula C16H12Cl2N2O
Molar Mass 319.19 g·mol−1
Formulation Neat Solid
Solubility
- DMF: 30 mg/ml
- DMSO: 30 mg/ml
- DMSO:PBS(pH7.2) (1:1): 0.5 mg/ml
- Ethanol: 10 mg/ml
Shipping & Storage Information
2′-Chlorodiazepam, Ro 5-3448, 2′-chloro-diazepam
Diclazepam is a benzodiazepine that was first synthesized in 1960 by Leo Sternbach and his colleagues at the Hoffman-La Roche research laboratory. It is not currently approved to be used as a medication. Diclazepam is a novel depressant substance that’s commonly used by benzodiazepine users. It exhibits the same effects as diazepam. Similar to diazepam, it has the ability to suppress anxiety and disinhibition. Its potency is around ten times that of diazepam.
In animal models, diclazepam exhibits a variety of effects, including long-acting anxiolytic properties and anticonvulsant properties.
Metabolism
The metabolism of diclazepam was studied to determine its elimination half-life. It was revealed that the compound undergoes N-demethylation and can be detected in urine for up to 6 days following administration.
Chemistry
Diclazepam is a benzodiazepine drug that has a seven-membered ring with two nitrogen constituents. It is commonly used as a replacement for methyl group. This drug’s seven-member diazepine ring is fused to a diazepine ring. It has a benzene ring and two nitrogen constituents. In addition, its phenyl ring is bonded to a 2-chlorinated phenyl ring.
It also has an oxygen group double bonded to its diazepine ring. This feature helps in forming a ketone.
Pharmacology
A benzodiazepine is known to produce various effects by binding to the site of the benzodiazepine receptor. The site is the most prolificly used inhibitory receptor in the brain. It enhances the effects of the gammabutyric acid on the brain. Diclazepam has a half-life of about 42 hours. It can be detected in urine for up to 6 days following the administration of parent compound. Other metabolites include lormetazepam and lorazepam.
Although benzodiazepines have anticonvulsant properties, they may be caused by the binding of voltage-dependent sodium channels instead of the benzodiazepine receptors.
Not much is known about the pharmacological and toxicological properties of this chemical. Usage of this Chemical should be for research and forensic purposes only.
WARNING This product is not for human or veterinary use.

This product is only available to persons of 21 years old and above.
Hazard statement(s)
| H302 | Harmful if swallowed |
| H315 | Causes skin irritation |
| H319 | Causes serious eye irritation |
| H332 | Harmful if inhaled |
| H335 | cause respiratory irritation |
| H336 | cause drowsiness or dizziness |
| Precautionary statement(s) | |
| P264 | Wash hands thoroughly after handling |
| P280 | protective gloves/protective clothing/eye protection/face protection |
| P305 + P351 + P338 | IF IN EYES: Rinse cautiously with for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. |
| P337 + P313 | If eye irritation persists: Get medical advice/attention |
| P261 | Avoid breathing dust/ fume/ gas/ mist/ vapors/ spray |
| P271 | Use only outdoors or in a well-ventilated area |
| P304 + P340 | IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing |
| P312 | Call a POISON CENTER or doctor/physician if you feel unwell |
| P403 + P233 | Store in a well-ventilated place. Keep container tightly closed |
| P405 | Store locked up |
| P501 | Dispose of contents/container to a licensed disposal company |




Reviews
There are no reviews yet.