Description
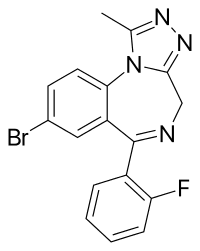
Flualprazolam
Product information
IUPAC-name 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-benzo[f] [1,2,4]triazolo[4,3-a] [1,4]diazepine
Synonyms Flualprazolam,
Formal name 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-benzo[f] [1,2,4]triazolo[4,3-a] [1,4]diazepine
Cas number. 28910-91-0
Formula C17H12ClFN4
Molar Mass 326.76 g·mol−1
Purity 99.0 % min.
Formulations A neat solid, Powder
Solubility
- DMF: 30 mgmL
- DMF:PBS(pH 7.2)(1:1): 0.5 mg/mL
- DMSO: 20 mg/mL
- Ethanol: 10 mg/mL
- Methanol: 1 mg/ml
Flualprazolam is a derivative of the TBZD class of drugs, which are commonly used as tranquilizers. It was first synthesized in 1976, but was never marketed. In 2018, it was identified as a designer drug in Sweden. It has similar anxiolytic and sedative effects to those of other drugs. It can be described as a 2′-fluoro derivative of alprazolam.
Flualprazolam is a fluorinated version of alprazolam, which has a central nervous system depressive effect. Although its synthesis was made in 1976, it was never marketed as a drug for treating depression.
Chemistry
Flualprazolam is a fluorine-based triazolobenzodiazepine that has the fluorine atom in the alprazolam’s phenyl ring. The IUPAC name of this drug is 8-chloro-6-methyl-4H-[1,2,4]triazolo[4,3-a],benzodiazepine. It has a CAS number of 28910-91. Flualprazolam’s molecular formula is C17H12ClFN4, and its molecular weight is 326.75 g/mol.
It is an off-white powder, which can be soluble in methanol and dichloromethane, and is also partially soluble in water. Like other benzodiazepines, such as triazolam and alprazolam, it shares a similar structure.
Synthesis
In the 1960s, a drug known as flualprazolam was synthesized. Although it was patented by Hester in 1976, it was never marketed as a medicinal product. The drug’s synthesis was carried out by refluxing a mixture of various chemicals, including 7-chloro-1,3,5-dihydro-5-(o-fluorophenyl)-2,benzodiazepine-2,thione, and acetic acid hydrohydrazide.
The solvent was extracted using dichloromethane. The organic layer of the residue was then dried over anhydrous sodium sulfate. After crystallization, the resulting flualprazolam was produced.
According to Fustero et al., an alternative method was used to produce flualprazolam. It involved treating N-nitrosoamidine with acetylhydrazine, which is the same chemical as amidine. After being treated with acetylhydrazine, the resulting amidine was then converted into a dimethylformamide.
The reaction was quenched with sodium bicarbonate after it cooled down at room temperature. The resulting organic layer was then dried and subjected to flash chromatography. After crystallization, it was then produced as flualprazolam.
Pharmacology and Toxicology
There is currently insufficient evidence supporting the safety and efficacy of flualprazolam in humans. Due to its structure, it is expected that this drug will have similar pharmacokinetics to other drugs such as triazolam and alprazolam.
In vitro studies have been conducted on the metabolism of flualprazolam using human liver microsomes. One of the two monohydroxylated and one dihydroxylated compounds were formed by the hydroxylation of the triazolo ring’s C4 diazepine core. In another study, the metabolic patterns of flualprazolam were studied by exposing the drug to different concentrations of pHLS9.
Six metabolites were detected during the various phases of the metabolism of flualprazolam. Three of these were derived from phase I and three from phase II reactions. A fourth phase II metabolite was also detected in a urine sample taken from an intoxicated patient. According to a study conducted by Sofalvi et al., -hydroxyflualprazolam is the main metabolite of flualprazolam.
The presence of different isoenzymes in the reactions was analyzed using single enzyme incubations. The three metabolites were only detected in the presence of CYP3A4, suggesting that the activity of this enzyme is mainly involved in the phase I reactions. Only two of the six metabolites were detected in the incubations with different concentrations of CYP2B6, CYP2C19, and CYP3A5. The glucuronide of the parent drug was also analyzed.
The seventh metabolite, which was only detected in the urine sample, was the -hydroxyflualprazolam glucuronide. In single enzyme incubations, the activity of UGT1A4, UGT2B10 and UGT2B4 was revealed to catalyze the formation of these compounds. The first two are involved in the N-glucurondation of flualprazolam, while the last one is responsible for the -hydroxyflualprazolam glucuronide’s catalytic activity.
In a real-world setting, only two of the seven metabolites of flualprazolam were detected in a plasma sample. On the other hand, all of the compounds and the parent drug were found in the urine samples.
The presence of N-glucuronide and the monohydroxylated metabolites of flualprazolam in urine samples has been suggested as possible screening targets for toxicological analysis. However, there is currently no published data on the activities of the flualprazolam metabolites.
What’s the difference between flualprazolam and alprazolam?
According to some users, flualprazolam is also stronger than other benzodiazepines, such as alprazolam. Users noted that this drug is 3–4 times more potent than alprazolam.
The toxicological and physiological properties of this compound has not been analyzed. Usage of this Chemical should be for research and forensic purposes only.
WARNING This product is not for human or veterinary use.

This product is only available to persons of 21 years old and above.
Hazard statement(s)
| H302 | Harmful if swallowed |
| H315 | Causes skin irritation |
| H319 | Causes serious eye irritation |
| H332 | Harmful if inhaled |
| H335 | cause respiratory irritation |
| H336 | cause drowsiness or dizziness |
| Precautionary statement(s) | |
| P264 | Wash hands thoroughly after handling |
| P280 | protective gloves/protective clothing/eye protection/face protection |
| P305 + P351 + P338 | IF IN EYES: Rinse cautiously with for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. |
| P337 + P313 | If eye irritation persists: Get medical advice/attention |
| P261 | Avoid breathing dust/ fume/ gas/ mist/ vapors/ spray |
| P271 | Use only outdoors or in a well-ventilated area |
| P304 + P340 | IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing |
| P312 | Call a POISON CENTER or doctor/physician if you feel unwell |
| P403 + P233 | Store in a well-ventilated place. Keep container tightly closed |
| P405 | Store locked up |
| P501 | Dispose of contents/container to a licensed disposal company |



Reviews
There are no reviews yet.