Description
N-Ethyl-Hexedrone HCI
Product information
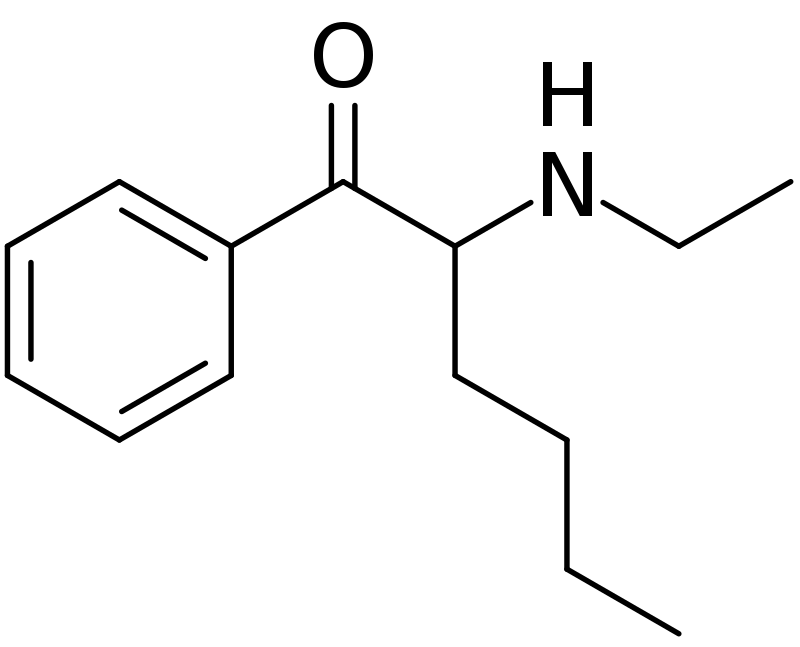
IUPAC-name 2-(Ethylamino)-1-phenylhexan-1-one mono
Synonyms α-ethylaminocaprophenone, N-ethylamino-Hexanophenone, N-ethylnorhexedrone, hexen, NEH
Formal name 2-(ethylamino)-
Cas number 18410-62-3
Formula C14H21NO • HCl
Formula Weight 255.8
Formulation A neat solid
Solubility
- DMF: 20 mg/ml
- DMSO: 10 mg/ml
- Ethanol: 25 mg/ml
- PBS (pH 7.2): 10 mg/ml
Shipping & Storage Information
N-Ethylhexedrone which is also known as α-ethylaminocaprophenone, N-ethylamino-Hexanophenone, N-ethylnorhexedrone, hexen, and NEH is a stimulant under the cathinone family. It acts as a norepinephrine-dopamine reuptake inhibitor with an IC50 values of 0.0978 and 0.0467 μM. N-Ethylhasedrone is a novel drug that’s commonly used as a cathinone class stimulant. It has little known about its pharmacology, but it likely increases levels of norepinephrine and/or dopamine in the brain.
N-Ethylhexedrone was first introduced in 1964. It is a novel psychoactive substance that was first created to mimic the effects of psychoactive substances. N-ethylhexedrone is a synthetic cathinone that has the same euphoric effects as cocaine and α-PVP.
Chemistry
N-Ethylexedrone is a derivative of the nitrogen atom’s methyl group, which is substituted by an acetyl group. It is similar to the concept of hexedrone. A substituted cathinone is a broad class of compounds that are mainly made up of the amphetamine core and its oxygen group. The formation of hundreds of possible compounds can be done through the modification of the cathinone backbone. This process involves adding substituent compounds to the aromatic ring or the alpha carbon.
N-ethylhexedrone has two added substitutions. The first is a n-butyl group, which is attached to amine group at RN2. The second one is an ethyl group.
Pharmacology
Synthetic cathinones are known to exert their effects by increasing the concentration of catecholamines in the brain. They can also inhibit the reuptake of monoamines and cause the release of biogenic amines. Currently, their pharmacological properties are not known. Based on its structure, N-ethylhexedrone is likely to be metabolized through N-dealkylation or reduction of the carbonyl group.
Synthetic cathinones are less able to cross the blood-brain barrier than amphetamines due to the beta-keto group’s increased polarity. Also, unlike dextroamphetamines, pyrrolidine derivatives have a low polarity.
The toxicological and physiological properties of this compound has not been analyzed. Usage of this Chemical should be for research and forensic purposes only.
WARNING This product is not for human or veterinary use.

This product is only available to persons of 21 years old and above.
Hazard statement(s)
| H302 | Harmful if swallowed |
| H315 | Causes skin irritation |
| H319 | Causes serious eye irritation |
| H332 | Harmful if inhaled |
| H335 | May cause respiratory irritation |
| H336 | May cause drowsiness or dizziness |
| Precautionary statement(s) | |
| P264 | Wash hands thoroughly after handling |
| P280 | Wear protective gloves/protective clothing/eye protection/face protection |
| P305 + P351 + P338 | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. |
| P337 + P313 | If eye irritation persists: Get medical advice/attention |
| P261 | Avoid breathing dust/ fume/ gas/ mist/ vapors/ spray |
| P271 | Use only outdoors or in a well-ventilated area |
| P304 + P340 | IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing |
| P312 | Call a POISON CENTER or doctor/physician if you feel unwell |
| P403 + P233 | Store in a well-ventilated place. Keep container tightly closed |
| P405 | Store locked up |
| P501 | Dispose of contents/container to a licensed disposal company |



Reviews
There are no reviews yet.